
Precautions for the use of NHS (N-hydroxysuccinimide)
In the field of bioresearch, the use of NHS (H-Hydroxysuccinimide: active esters) is the only option for the reaction of carboxylic acids with amines, and there appears to be no concept of mixing a dehydrating agent with the precursor carboxylic acid to react with the amine.
On the other hand, for researchers in organic chemistry, NHS is nothing special and only an option: if NHS is available commercially at low cost, they will use it without hesitation, otherwise they will not bother to purify carboxylic acids by leading them to NHS, but will use the carboxylic acid + dehydrating agent combination as in Fig.4 below. The combination of carboxylic acid + dehydrator is a one-pot reaction, as shown in Fig. 4 below.
NHS Advantages.
- The desired amide bond is formed simply by mixing NHS with the amine component. No particularly difficult technology is required.

- There is no risk of side reactions even if another carboxylic acid is present in the reaction system.
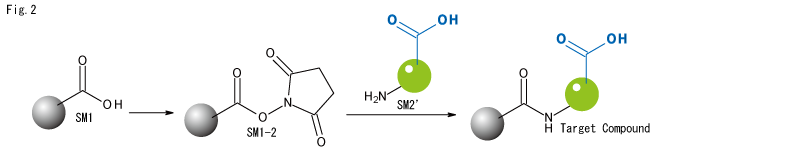
Drawbacks of the NHS
- Generally more expensive than carboxylic acids.
- If the NHS can be separated from other substances by precipitation using solvents such as diethylether, no problem occurs, but if not, purification is difficult due to its structure, which is prone to hydrolysis. Partial hydrolysis during column purification is inevitable and the product is usually left unpurified for the next amidation step.
- There are concerns about storage stability as it reacts with trace amounts of water and hydrolyses. In particular, once opened, the product will gradually decompose due to moisture in the air.
Indeed, LC-MS analysis of commercial NHS compounds in reaction with large excesses of amine compounds often shows small amounts of carboxylic acids where the NHS has been hydrolysed.
Cases where it is easier to use the NHS
- If another carboxylic acid is present in the molecule and more dehydrating agent is used, side reactions can occur where the undesirable carboxylic acid is activated by the excess dehydrating agent and reacts with the amine. The by-products created by this can further react with the excess dehydrator to form complex oligomeric mixtures, as the by-products also have amines and carboxylic acids.
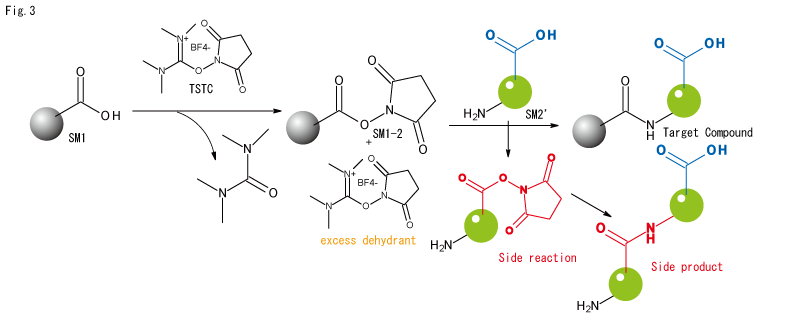
Cases where carboxylic acid + dehydrating agent is sufficient without NHS.
- All other than above. Simply mix the dehydrator. You do not need to worry about the order in which they are added.
- Even in the above case, the side reaction can be avoided by making it a one-pot, two-step reaction, using a dehydrating agent (TSTU in the diagram but a common dehydrating agent: WSC, BOP, whatever) at exactly 1:1 (or slightly less than the carboxylic acid) and sampling before adding step 2: the amine component SM2'. and carry out an NHS reactivity check before adding step 2: amine component SM2' to avoid side reactions. We have had many successes in this case.
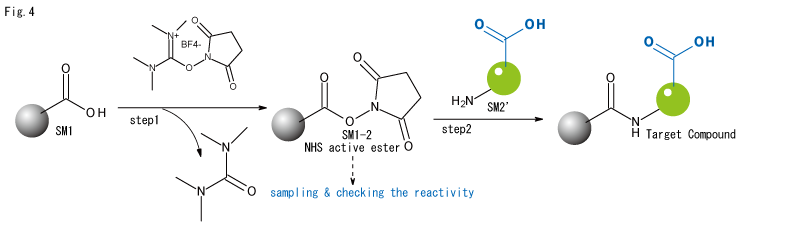
Special cases
- Rarely, but not always, when another carboxyl group is contained in the same molecule of a carboxylic acid component (SM1') and selectivity of the amide bond position is required,
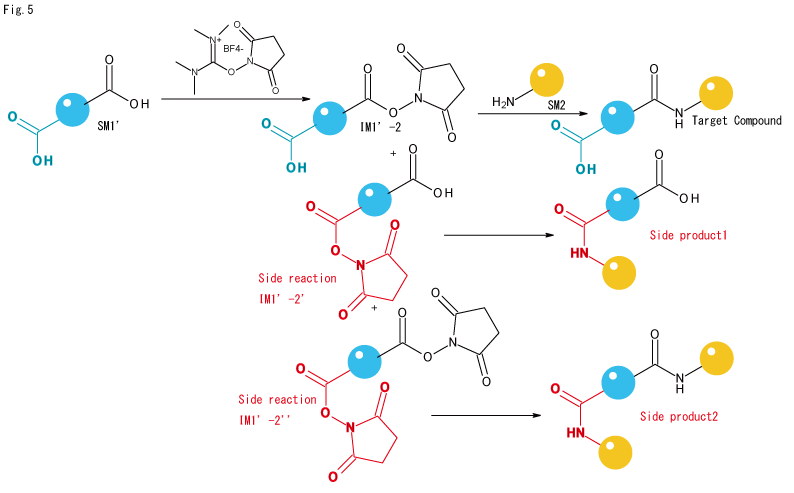
- If there is a commercially available product with a structure like IM1'-2, in which only the desired carboxyl group is selectively NHSylated, there is no alternative but to buy this.
- If no commercially available product can be found, one option is to forcefully separate SideProducts 1 and 2 from the reaction solution obtained by the procedure shown in Fig. 5 using HPLC. However, if separation conditions for isomers with different bonding positions cannot be found, the mixture must be used as is, and even if it can be separated, structural analysis to confirm that the target carboxylic acid is activated may not be easy.
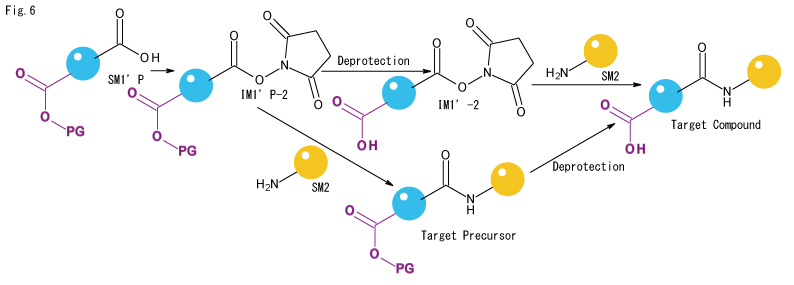
- If 100% selectivity is the goal, the method shown in Fig. 6 is adopted, although the synthesis is much more time-consuming. Normally, the PG of the Target Precursor is deprotected after binding IM1'P-2 to the amine component, but if the Precursor or Target Compound degrades under deprotection conditions, another protecting group should be considered, or deprotection should be carried out first and the The IM1'-2 structure is isolated once on the column and then reacted with the amine component. In the latter case, partial hydrolysis during the isolation process is inevitable.
When you request delivery with NHS (N-Succinimidyl Active Ester) instead of carboxylic acid, we will do the following
- Equimolar dehydrating agents are used to bring the carboxylic acid to NHS.
If it seems possible to purify by precipitation, the reaction solution is dropped into a poor solvent, centrifuged or filtered, dried and partly used to check reactivity with amines before delivery. - If the NHS of interest is not crystalline and does not precipitate, column purification (silica gel or ODS) can be attempted at the customer's request. We will use a portion of the dried product of the purified fraction to check the reactivity with amines before delivery. Please note that partial hydrolysis after purification is inevitable.
- If it is found that severe hydrolysis during column purification is unavoidable, column purification is omitted. A portion of the reaction solution is used to check the reactivity with amines and then the solvent is removed under reduced pressure and the dried product is delivered. Please note that dehydrating agent debris (which does not affect the reaction with amines) will be included.
- If it is clear that there are no other carboxylic acids in the reaction system, we use a slightly excessive amount of dehydrating agent relative to the raw carboxylic acid, even if the product is delivered unpurified. (This will result in a reaction rate closer to 100% with the amine component.)
- If there are two or more carboxylic acids in the same molecule and selectivity is required, the IM1'-2 structure shown in Fig. 6 should be delivered, but we expect that the synthesis of SM1' will require a very tedious process, so please let us discuss the cost.
Click here  for custom synthesis of organic compounds.
for custom synthesis of organic compounds.
Click here for any Orgenic compounds.
for any Orgenic compounds.
Compound search:Set keywords and then click SEARCH.
Compound search:Check categories and click SEARCH button.
