
Active sulphur molecules.
Active sulphur molecules such as cysteine polysulphide, to which excess sulphur atoms are added to the thiol (SH) group of cysteine,
are abundant in living organisms and are known to have ponent antioxidant and redox signalling regulatory properties.
We have established an efficient synthesis method for oxidised active sulphur molecules (polysulphides).
We have isolated each high purity product by HPLC purification of a mixture of thiols (n=1) and sulphur insertions (n=3-8).
We have experience in the synthesis of cysteine and glutathione, and the same synthetic method can be applied to other common thiol-containing compounds as starting materials.
Please feel free to contact us.
us.
| SCHEM No. | Structure | Compound Name | Price (JPY) | Purity | Stock(mg) |
|---|---|---|---|---|---|
| 07609 | 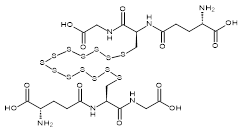 | Glutathione tridecasulfide | 170,000/0.1mg | <80% | 0.7 |
| 07608 | 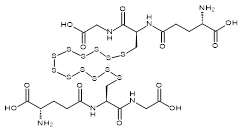 | Glutathione dodecasulfide | 170,000/0.1mg | <80% | 0.6 |
| 07607 | 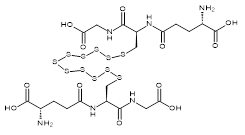 | Glutathione undecasulfide | 170,000/0.1mg | <80% | 0.7 |
| 07606 | 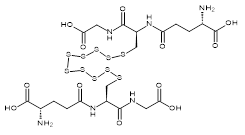 | Glutathione decasulfide | 170,000/0.3mg | <80% | 0.9 |
| 07603 | 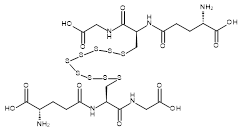 | Glutathione nonasulfide | 170,000/0.5mg | >80% | 1.5 |
| 07524 | 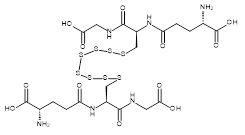 | Glutathione octasulfide | 170,000/0.8mg | >90% | 1.7 |
| 06866 | 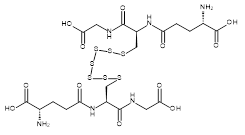 | Glutathione heptasulfide | 170,000/1mg | >90% | 3.2 |
| 06865 | 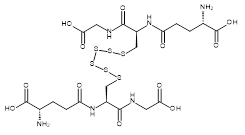 | Glutathione hexasulfide | 170,000/2mg | >90% | 9.9 |
| 06862 | 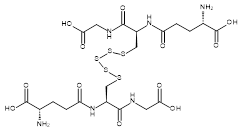 | Glutathione pentasulfide | 170,000/5mg | >90% | 22.8 |
| 06863 | 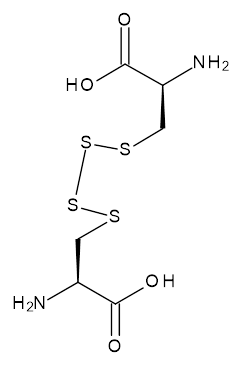 | L-Cysteine tetrasulfide | 69,600/0.1mg | >95% | 0 |
| 06861 | 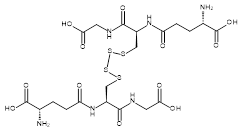 | Glutathione tetrasulfide | 170,000/10mg | >90% | 17.0 |
| 06860 | 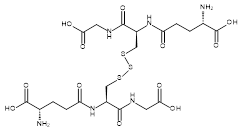 | Glutathione trisulfide | 170,000/30mg | >90% | 99.5 |
Compounds not listed may be available at similar prices. If the structure has only different substituents, the synthesis method has already been established, so it takes 1-2 weeks and can be synthesised in the same level price range.
Compound search:Set keywords and then click SEARCH.
Compound search:Check categories and click SEARCH button.
